Evidence and Efficacy
Defense Against Candida auris Superbug
Candida auris is a deadly superbug first identified in 2009, and it has rapidly become an emerging global threat to patient health. It is often highly resistant to multiple major antifungal drug classes, and the reported mortality rate for C. auris infections is between 30% and 60%. Following several consecutive years of rapid annual increases in U.S. clinical prevalence (2018-2019: 44% increase, 2019-2020: 59% increase, 2020-2021: 95% increase), C. auris is a grave and growing concern for patients, clinicians, and infection preventionists.
PrevahexCHX® and competitive dressings were tested for their ability to inhibit growth of C. auris in an in vitro zone of inhibition study. Dressing samples (10mm x 10mm) were placed directly into petri dishes of growth media which had been pre-inoculated with C. auris (ATCC# MYA-5001). Samples were incubated for 1 day before zone size was measured for each article. This study was conducted at an independent, contract laboratory under GLP and each dressing type was tested in quadruplicate.*
*Note: In vitro effectiveness does not predict clinical performance. (Data on File: entrotech life sciences, llc, Columbus, OH)
Summary of Results from Comparative Dressing Test Against Candida auris:
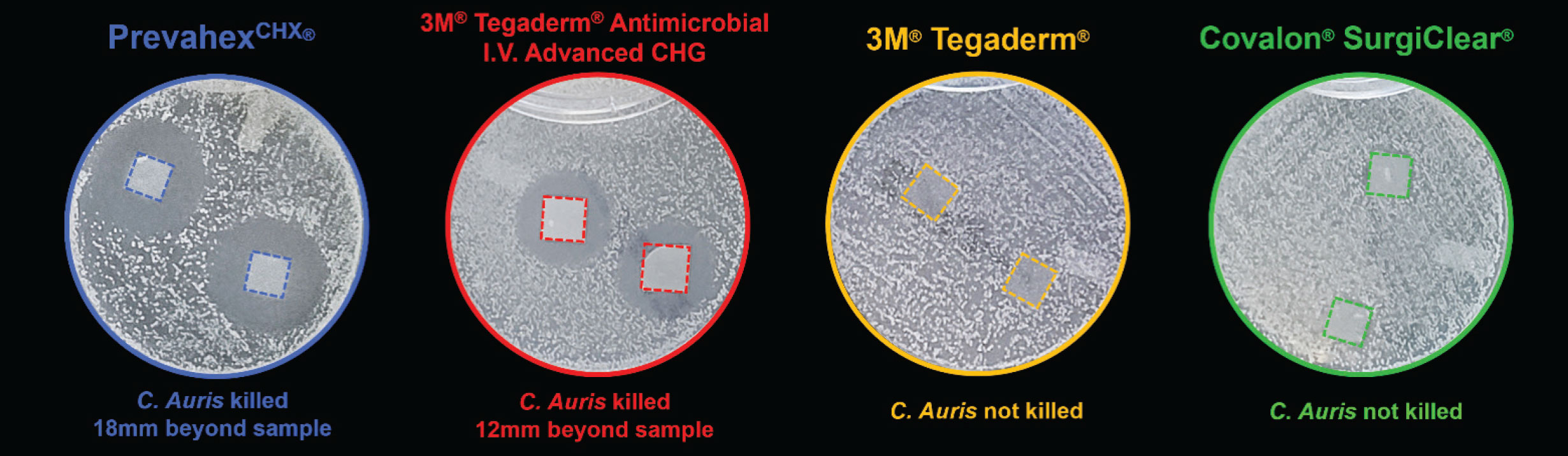
- PrevahexCHX® demonstrated a zone of inhibition 50% wider than Tegaderm® Antimicrobial I.V. Advanced CHG
- PrevahexCHX® provided an area of protection 90% larger than Tegaderm® Antimicrobial I.V. Advanced CHG
- Tegaderm® and Covalon® SurgiClear® showed no efficacy
For the Summary of Results from Comparative Dressing Test Against Candida auris, download the brochure.
SurgiClear® is a registered trademark of Covalon Technologies Ltd.; Tegaderm is a registered trademark of Solventum Intellectual Properties Company.